
Using our newly acquired fully automated Olympus BX63 Microscope, OSIMAP team members are able to quickly image microplastics in great detail. Many microplastic particles are small, and can be difficult to distinguish from other naturally occurring particles such as organic material, plankton and sediments, using a microscope is an essential first step in our assessement of microplastic type, size and abundance.
Our environmental samples (filtered onto membrane filters) can be viewed in brightfield (white light) or any of 3 different fluorescent light channels (GFP, Texas Red, DAPI). We use fluoresence, as plastic particles, when dyed with special stains can stand out amongst other particles on the filter, allowing our team to count, measure and eventually determine their composition. Our microscope allows us to automatically produce images of all our channels quickly, eventually stacking the layers on top of one another to create composites.
An entire filter membrane can also be scanned in simply by indicating on the software what areas should be imaged. This can be indicated on a map of the entire sample that can be acquired by the microscope within a few minutes after the “acquire overview” button is pressed. Even if the sample is not completely flat, a z-stack can be used to keep the entire depth of a portion of the filter in focus. The z stack is made up of a series of pictures that range from the highest portion of a sample to the bottom so they can be combined into one focused image. After the scan of the filter is done (15-20 minutes later), the software can also be programmed to identify and count the number of microplastics on the filter. The counted particles can then be reviewed by a lab member who will remove any particles from the count that were incorrectly counted as a plastic. All of this is usually done with the 4x objective lens, but 10x and 40x lens are available if a closer inspection is needed beyond what the zoom function on the pictures can provide.
Seawater samples must be filtered onto a polycarbonate filter membrane before they can be imaged. Seawater that appears clear can be filtered immediately on polycarbonate filter membranes while the seawater at the bottom of sample jars (which usually contains some sediment and seaweed) must be put through alkali digestion. It also goes through density separation if the sample has too much sediment. The alkali digestion dissolves organic matter while the density separation causes the plastic to float and the sediment to sink. The result is a single layer of material on the filter membrane that can all be scanned into the microscope. The filter membrane is then stained with nile red so the microplastic will fluoresce green, red, or yellow (depending of polymer type). Calcoflour white and Evans blue is then applied to both stop Nile red fluorescence in biological material and cause the biological material to fluoresce blue or purple.
All of these features combined give the OSIMAP team the capacity to rapidly count how many microplastics are in each of their samples. The team is still in the starting stages of leaning to use the Olympus microscope effectively, but it will be an invaluable tool once the imaging process is optimized. The plastics also need to be put through Raman microscopy before their exact composition can be figured out.
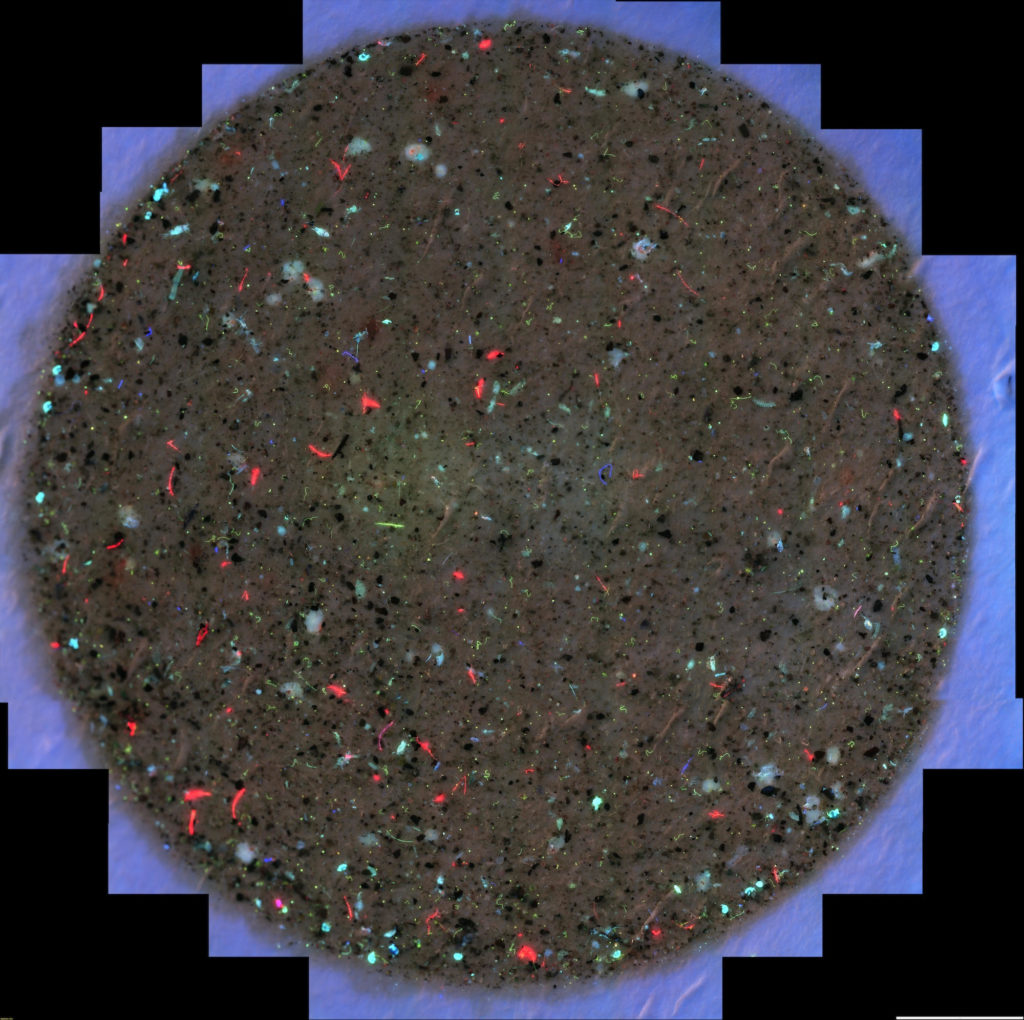
Scan of a filter membrane with some of the the material caught on a 100 um filter after 20 gallons of Narragansett Bay water was pumped through it. All 3 channels of fluorescence (GFP, Texas Red, and DAPI) are visible at the same time in this scan. The fluorescent particles are pieces of plants, animals, and plastics. The brown background is sediment. This filter has not been stained so all fluorescence seen here is auto-fluorescence.
